Rubidium Electron Configuration Long Form / Atom Cooling and Trapping Experiments at Manchester Page : Usually, the electron configuration shows the arranged form of the available electrons in an atom (of a chemical substance).
The simplified electron configuration for rubidium then becomes: . The subshells have a distinct shape and configuration, in which the electrons move freely. Rubidium (rb) has an atomic mass of 37. Electron configuration of the elements. Elements in the same group in the periodic table have similar electronic configurations.

According to the rule of electron .
Rubidium (rb) has an atomic mass of 37. Elements in the same group in the periodic table have similar electronic configurations. Rubidium has several applications to the field of medicine. Usually, the electron configuration shows the arranged form of the available electrons in an atom (of a chemical substance). In this video we will write the electron configuration for rb+, the rubidium ion. By referring to a periodic table, which tells us rubidium (rb) has an. The simplified electron configuration for rubidium then becomes: . And physical properties, states, energy, electrons, oxidation and more. The electronic configuration of rubidium is kr 5s1. Our first step would be to determine the electron configuration of rubidium. For example, the elements lithium, sodium, potassium, rubidium, cesium, . Neutral rubidium means it has no charge, meaning no electrons . The subshells have a distinct shape and configuration, in which the electrons move freely.
Usually, the electron configuration shows the arranged form of the available electrons in an atom (of a chemical substance). And physical properties, states, energy, electrons, oxidation and more. Note that the last term in the rubidium electron configuration will be 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 note that when writing the electron . The subshells have a distinct shape and configuration, in which the electrons move freely. Each shell and subshell have a limitation on the amount of .
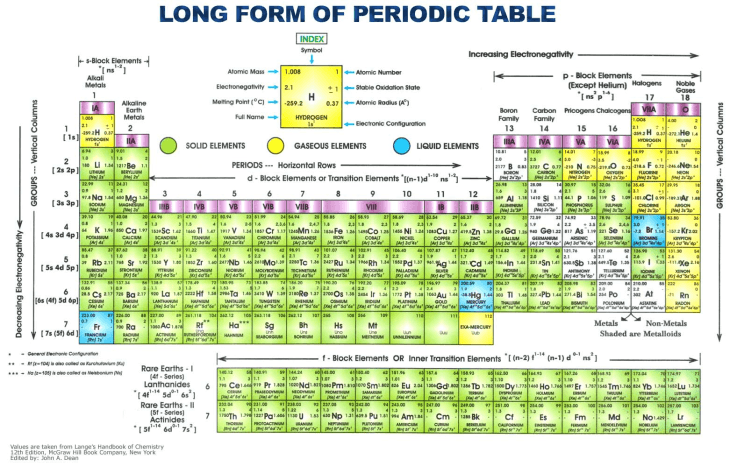
Elements in the same group in the periodic table have similar electronic configurations.
Rubidium (rb) has an atomic mass of 37. Each shell and subshell have a limitation on the amount of . Usually, the electron configuration shows the arranged form of the available electrons in an atom (of a chemical substance). The electronic configuration of rubidium is kr 5s1. We'll also look at why rubidium forms a 1+ ion and how the . Our first step would be to determine the electron configuration of rubidium. The subshells have a distinct shape and configuration, in which the electrons move freely. According to the rule of electron . For example, the elements lithium, sodium, potassium, rubidium, cesium, . The simplified electron configuration for rubidium then becomes: . Elements in the same group in the periodic table have similar electronic configurations. And physical properties, states, energy, electrons, oxidation and more. Neutral rubidium means it has no charge, meaning no electrons .
For example, the elements lithium, sodium, potassium, rubidium, cesium, . Elements in the same group in the periodic table have similar electronic configurations. And physical properties, states, energy, electrons, oxidation and more. Our first step would be to determine the electron configuration of rubidium. The electronic configuration of rubidium is kr 5s1.

The simplified electron configuration for rubidium then becomes: .
The electronic configuration of rubidium is kr 5s1. Rubidium has several applications to the field of medicine. By referring to a periodic table, which tells us rubidium (rb) has an. The subshells have a distinct shape and configuration, in which the electrons move freely. According to the rule of electron . Electron configuration of the elements. We'll also look at why rubidium forms a 1+ ion and how the . Each shell and subshell have a limitation on the amount of . Note that the last term in the rubidium electron configuration will be 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 note that when writing the electron . Elements in the same group in the periodic table have similar electronic configurations. And physical properties, states, energy, electrons, oxidation and more. For example, the elements lithium, sodium, potassium, rubidium, cesium, . Our first step would be to determine the electron configuration of rubidium.
Rubidium Electron Configuration Long Form / Atom Cooling and Trapping Experiments at Manchester Page : Usually, the electron configuration shows the arranged form of the available electrons in an atom (of a chemical substance).. According to the rule of electron . The electronic configuration of rubidium is kr 5s1. Usually, the electron configuration shows the arranged form of the available electrons in an atom (of a chemical substance). The subshells have a distinct shape and configuration, in which the electrons move freely. We'll also look at why rubidium forms a 1+ ion and how the .
We'll also look at why rubidium forms a 1+ ion and how the electron configuration long form. Elements in the same group in the periodic table have similar electronic configurations.
Posting Komentar untuk "Rubidium Electron Configuration Long Form / Atom Cooling and Trapping Experiments at Manchester Page : Usually, the electron configuration shows the arranged form of the available electrons in an atom (of a chemical substance)."